Weighing the Right Choice of Matter

Understanding Concept behind the Second task of Stage 4
Have you ever realized how much you have grown from when you were little? Right now, you are probably thrice your size from when you were a toddler. While it is more apparent to notice the weight of things in a large scale, do you know even the smallest bit of matter, the atom, also carries weight?
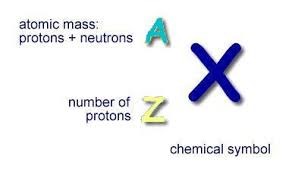
Atomic mass is measured by the sum of protons and neutrons in the nucleus of a certain atom. Because electrons weigh so little, they usually do not contribute to the atom’s mass. As we know, different atoms with different properties are classified as elements. Each of the 118 elements listed in the modern periodic table has their own corresponding atomic masses.
It must be known, however, the term Atomic Mass should not be confused with Atomic Weight. Atomic weight is the relative atomic mass of each element. Think of it as the ratio of the average mass of an element by also considering its isotopes. An isotope is a species of a certain atom that differs by the number of neutrons. For instance, do you know carbon has Though sometimes the Atomic mass and Atomic weight are used interchangeably just as seen in the examples below, it is important to recognize their difference.

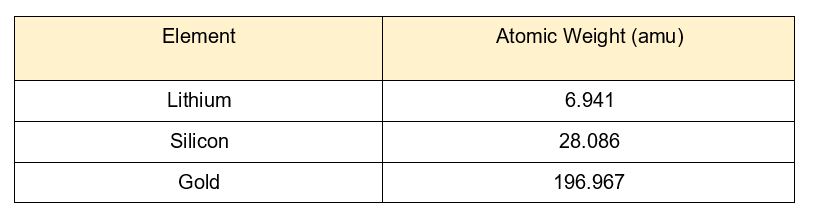
Atomic weight is measured in atomic mass units (amu), also called daltons. Here is a list of some elements with their atomic weights. (Tip: these are hints for the 4th stage so read them carefully!)
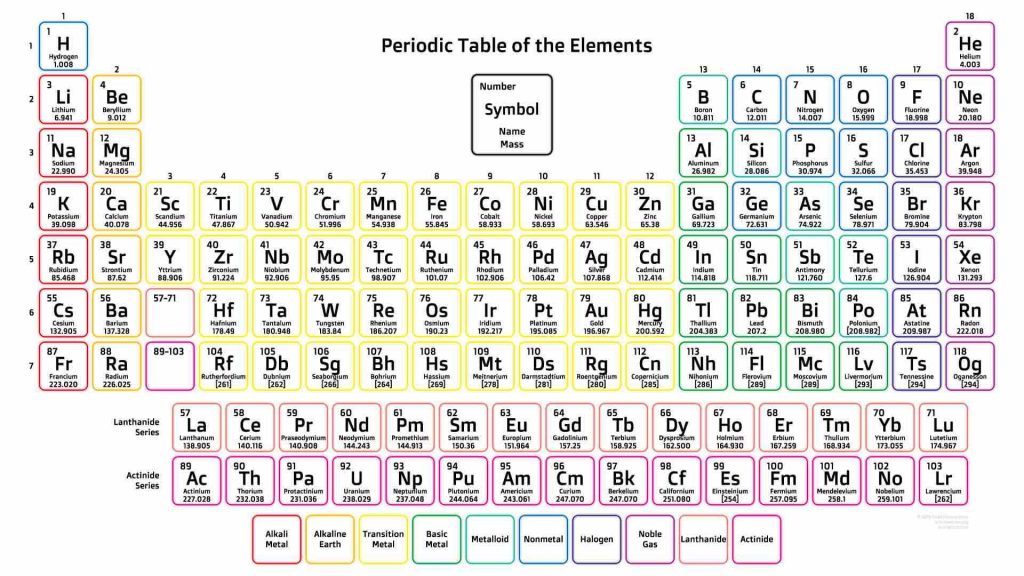
All of the known elements to man have their atomic weights not only identified, but also tactically arranged in the periodic table. Looking at the periodic table, you could have noticed that the atomic weight increases as atomic number increases. From left to right, the atomic weight increases. The same increasing trend is seen from top to bottom.
Since it is unpractical for scientists to count the number of particles in a given reaction, chemists will opt to weigh each of the atoms to scale their quantity. The atomic weight is a fundamental property of matter because it can greatly predict the behavior, reaction and tendencies of the atom which could help scientists arrive to quantifiable conclusions.
Sources:
https://www.remm.nlm.gov/atomicshorthand.htm
https://socratic.org/chemistry
https://www.thoughtco.com/color-periodic-table-with-atomic-masses-608859





Responses